Research activity
-
NMDA channels in native and recombinant receptors:
role of the different subunits in modulation by metal ions
The N-methyl-D-aspartate subtype of glutamate receptor is a very important receptor in the central nervous system, implicated in the mechanisms of synaptic plasticity and high brain functions, as well as in neurological and neurodegenerative diseases. This ligand-gated ionic channel carries the slow component of the glutamate-activated postsynaptic current and is endowed with numerous unique properties: it conducts monovalent cations, but has a significantly high calcium permeability, it is tonically blocked by magnesium (Mg2+) in a voltage-depedent manner and allosterically modulated by zinc (Zn2+), as well as by various other endogenous and exogenous ligands.
|
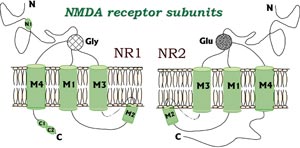
NMDA receptors contain two different types of
subunits, NR1 and at least one of the four
different NR2 types (A, B, C and D).
|
Divalent metal
ions can affect the NMDA channel activity in a voltage-dependent (Mg-like) or
voltage-independent (Zn-like) manner.
We have studied the effect of two toxic metals, lead (Pb2+) and nickel (Ni2+), in native receptor in cerebellar granule neuron from neonatal rats and in
recombinant NMDA channel expressed in RNA-injected Xenopus laevis oocytes or in
transiently transfected mammalian HEK293 cells.
Pb2+mediates a voltage-independent allosteric modulation in NMDA channels and its
inhibition is independent of the subinit present in the recptor (either NR2A
or NR2B). On the contrary, Ni2+
has a voltage-dependent Mg-like effect, but also causes a voltage-independent
inhibition of NR2A-containing receptor activity and a potentiation in
NR2B-containing receptor activity. This last effect is similar to
polyamine-mediated potentiation.